Nivolumab (Opdivo) demonstrates survival benefit in squamous and non-squamous non-small cell lung cancer (NSCLC)
Last Updated on October 11, 2015 by Joseph Gut – thasso
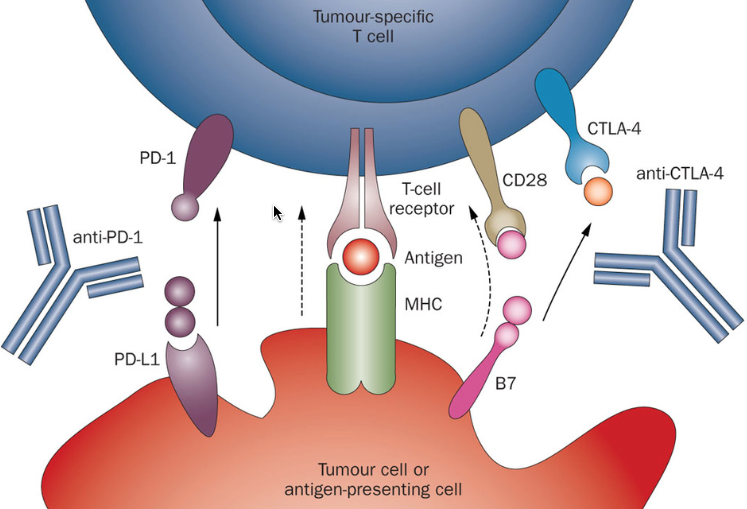
October 11, 2015 – In recent months, Nivolumab (Opdivo), a human IgG4 anti-PD-1 monoclonal antibody which targets the PD-1 receptor, had been approved first for the treatment of unresectable or advanced (metastatic) melanoma and secondly for the treatment of advanced (metastatic) squamous non-small cell lung cancer (NSCLC). This week, the American Food and Drug Administration approved Nivolumab (Opdivo) to also treat patients with advanced (metastatic) squamous non-small cell lung cancer whose disease progressed during or after platinum-based chemotherapy.
Lung cancer is one of the leading cause of cancer death in the US and worldwide. The most common type of lung cancer, non-small cell lung cancer (NSCLC), is further divided into two main types named for the kinds of cells found in the cancer, namely squamous cells and non-squamous cells (which includes adenocarcinoma). Nivolumab (Opdivo) works by targeting the cellular pathway known as PD-1/PD-L1 (proteins found on the body’s immune cells and some cancer cells). By blocking this pathway, Nivolumab (Opdivo) may help the body’s immune system fight the cancer cells. The approval of Nivolumab (Opdivo) earlier this year was for the treatment of patients with advanced squamous NSCLC whose disease progressed during or after platinum-based chemotherapy. The current approval now expands the use of Opdivo to also treat patients with non-squamous NSCLC.
The effectiveness of Nivolumab (Opdivo) for this indication was demonstrated in an international, open-label, randomized study of 582 participants with advanced non-squamous NSCLC whose disease progressed during or after treatment with platinum-based chemotherapy and appropriate biologic therapy. Participants were treated with Nivolumab (Opdivo) or docetaxel. The primary endpoint was overall survival, and the secondary endpoint was objective response rate (the percentage of  patients who experienced complete or partial shrinkage of their tumors). Those treated with Nivolumab (Opdivo) lived an average of 12.2 months compared to 9.4 months in those treated with docetaxel. Additionally, 19 percent of those treated with Nivolumab (Opdivo) experienced a complete or partial shrinkage of their tumors, an effect that lasted an average of 17 months, compared to 12 percent among those taking docetaxel, which lasted an average of six months.
patients who experienced complete or partial shrinkage of their tumors). Those treated with Nivolumab (Opdivo) lived an average of 12.2 months compared to 9.4 months in those treated with docetaxel. Additionally, 19 percent of those treated with Nivolumab (Opdivo) experienced a complete or partial shrinkage of their tumors, an effect that lasted an average of 17 months, compared to 12 percent among those taking docetaxel, which lasted an average of six months.
According to Richard Pazdur, M.D., director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, there remains still a lot to be learned about the PD-1/PD-L1 pathway and its effects in lung cancer, as well as other tumor types. Thus, while patients who received Nivolumab (Opdivo) lived longer than those who received docetaxel across the study, an evaluation of samples from a subgroup of patients’ tumors suggests that the level of PD-L1 expression in NSCLC tumors may help identify patients who are more likely to live longer due to treatment with Nivolumab (Opdivo). Therefore the FDA also approved the PD-L1 IHC 28-8 pharmDx test to detect PD-L1 protein expression levels and help physicians determine which patients may benefit most from treatment with Nivolumab (Opdivo).
Another drug called Pembrolizumab (Keytruda) also targets the PD-1/PD-L1 pathway. It was approved just last week for treating NSCLC specifically for patients whose tumors expressed PD-L1. Thasso Post had a recent report on Pembrolizumab (Keytruda).
As one might expect from immune checkpoint modulating drugs, Nivolumab (Opdivo) comes with a rather serious and complicated clinical safety profile, which in some patients simply may lead to therapy terminating events with sometimes fatal outcome. In an earlier article in Thasso Post, inclusive the comment section to this article, these clinical safety concerns with respect to Opdivo have substantiated.
Follow below a commentary by Corey Langer, MD, on the immune checkpoint therapy approach involving Nivolumab (Opdivo):


Hey very interesting blog!
whoah this weblog is excellent i love reading your
posts. Stay up the good work! You already know, a lot
of persons are searching around for this information, you could help them greatly.
What’s up mates, pleasant post and good urging commented at this place, I am
really enjoying by these.
Thanks for sharing your thoughts about nivolumab (opdivo).
Regards
You really make it seem so easy with your presentation but I find this
topic to be actually something that I think I would never
understand. It seems too complicated and extremely broad for me.
I am looking forward for your next post, I’ll try to get the hang
of it!
Genuinely when someone doesn’t understand then its up to
other visitors that they will help, so here it occurs.
On November 23, 2015, the U.S. Food and Drug Administration approved Nivolumab (Opdivo) to treat patients with advanced (metastatic) renal cell carcinoma, a form of kidney cancer, who have received a certain type of prior therapy.
“Opdivo provides an important therapy option for patients with renal cell carcinoma,” said Richard Pazdur, M.D., director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. “It is one of few therapies that have demonstrated the ability to extend patients’ survival in treating this disease.” In fact, Torisel (temsirolimus), approved in 2007, is the only other FDA-approved therapy that has demonstrated overall survival in renal cell cancer.
Renal cell carcinoma is the most common form of kidney cancer in adults and forms in the tissues of the kidney that make urine. The National Cancer Institute estimates 61,560 new cases and 14,080 deaths from kidney and renal pelvis cancer in the United States this year.
Opdivo’s extended indication, from melanoma and non-small cell lung cancer to renal cell cancer, demonstrates how immune therapies can benefit patients across a wide range of tumors,” continued Dr. Pazdur. Besides its efficacy in renal cell carcinoma, Opdivo comes with some adverse effects. The most common side effects of Opdivo for this use are conditions relating to abnormal weakness or lack of energy (asthenic conditions), cough, nausea, rash, difficulty breathing (dyspnea), diarrhea, constipation, decreased appetite, back pain and joint pain (arthralgia). Optivo has also has the potential to cause serious side effects that result from the immune system effect of Opdivo (known as “immune-mediated side effects”). These severe immune-mediated side effects involve healthy organs, including the lung, colon, liver, kidneys, hormone-producing glands and the brain.
Read the full announcemebt here:
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm473971.htm