First therapy for children with debilitating NF-1 approved in the USA
Last Updated on April 14, 2020 by Joseph Gut – thasso
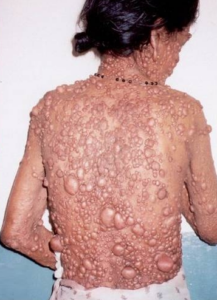
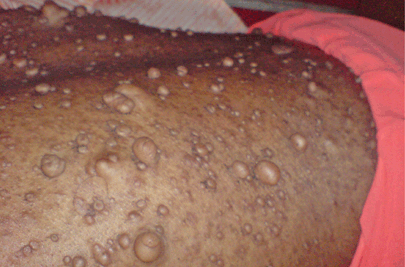
In the midst of its occupany with the rise of Covid-19, the American Food and Drug Administration (FDA) just approved Selumetinib (Koselugo) for the treatment of pediatric patients, 2 years of age and older, who suffer from debilitating, progressive, and often disfiguring yet rare neurofibromatosis type 1 (NF-1), which typically begins early in life. In fact, NF-1 is an age specific disease; most signs of NF-1 are visible after birth (during infancy), but many symptoms of NF-1 occur as the person ages and changes hormonal status. NF-1 is usually diagnosed in early childhood and appears in an estimated 1 out of every 3,000 infants. As of 2015, there were at least 100,000 people in the U.S. and about 15,000 people in the UK who had been diagnosed with NF. Common symptoms of NF-1 include brownish-red spots in the colored part of the eye called Lisch nodules, benign skin tumors called neurofibromas, and larger benign tumors of nerves called plexiform neurofibromas (PN).
Here, Selumetinib (Koselugo) is approved specifically for patients who have symptomatic, inoperable plexiform neurofibromas, which are tumors involving the nerve sheaths (coating around nerve fibers) and can grow anywhere in the body, including the face, extremities, areas around the spine and deep in the body where they may affect organs. Selumetinib (Koselugo) is a kinase inhibitor, meaning it functions by blocking a key enzyme, which results in helping to stop the tumor cells from growing. With this approval, for the first time, pediatric patients now have an FDA-approved drug to treat plexiform neurofibroma.
The FDA approved Selumetinib (Koselugo) based on a clinical trial conducted by the National Cancer Institute of pediatric patients who had NF-1 and inoperable PN (defined as a PN that could not be completely removed without risk for substantial morbidity to the patient). The efficacy results were from 50 of the patients who received the recommended dose and had routine evaluations of changes in tumor size and tumor-related morbidities during the trial. Patients received Selumetinib (Koselugo) 25 mg/m2 orally twice a day until disease progression or until they experienced unacceptable adverse reactions. The clinical trial measured the overall response rate (ORR), defined as the percentage of patients with a complete response and those who experienced more than a 20% reduction in PN volume on MRI that was confirmed on a subsequent MRI within 3-6 months. The ORR was 66% and all patients had a partial response, meaning that no patients had complete disappearance of the tumor. Of these patients, 82% had a response lasting 12 months or longer. Other clinical outcomes for patients during Selumetinib (Koselugo) treatment including changes in PN-related disfigurement, symptoms and functional impairments. Although the sample sizes of patients assessed for each PN-related morbidity (such as disfigurement, pain, strength and mobility problems, airway compression, visual impairment and bladder or bowel dysfunction) were small, there appeared to be a trend of improvement in PN-related symptoms or functional deficits during treatment.
Of course, patients and treating physicians need to be concerned about common and serious side effects of Selumetinib (Koselugo) too. Common effects included vomiting, rash, abdominal pain, diarrhea, nausea, dry skin, fatigue, musculoskeletal pain (i.e., pain in the body affecting bones, muscles, ligaments, tendons and nerves), fever, acneiform rashes (acne), stomatitis (inflammation of the mouth and lips), headache, paronychia (infection in the skin that surrounds a toenail or fingernail) and pruritus (itching). However, much more seriously, Selumetinib (Koselugo) can also cause effects such as heart failure (manifested as ejection fraction decrease, or when the muscle of the left ventricle of the heart is not pumping as well as normal) and ocular toxicity (acute and chronic damage to the eye) including retinal vein occlusion, retinal pigment epithelial detachment and impaired vision. Patients should have cardiac and ophthalmic assessments performed prior to initiating Selumetinib (Koselugo) and at regular intervals during treatment. Selumetinib (Koselugo) can also cause increased creatinine phosphokinase (CPK). CPK is an enzyme found in the heart, brain and skeletal muscles. When muscle tissue is damaged, CPK leaks into a person’s blood. CPK elevation in a patient receiving Selumetinib (Koselugo) should prompt an evaluation for rhabdomyolysis (breakdown of skeletal muscle due to direct or indirect muscle injury). Selumetinib (Koselugo) should be withheld, dosage reduced or dosage permanently discontinued based on the severity of adverse reactions. In addition, Selumetinib (Koselugo) contains Vitamin E, and patients are at an increased risk of bleeding if their daily intake of Vitamin E exceeds the recommended or safe limits.
Finally and very much disturbingly, based on findings from animal studies, Selumetinib (Koselugo) may cause harm to a newborn baby when administered to a pregnant woman. The FDA advises health care professionals to tell females of reproductive age, and males with female partners of reproductive potential, to use effective contraception during treatment with Selumetinib (Koselugo), and for one week after the last dose. See here the FDA-approved drug label for Selumetinib (Koselugo).
See here a short sequence on neurofibromatosis type 1 (NF-1):

