New Treatment Modality: Nivolumab (Opdivo) has been approved for the treatment of patients with previously treated metastatic squamous non-small cell lung cancer (NSCLC)
Last Updated on June 17, 2015 by Joseph Gut – thasso
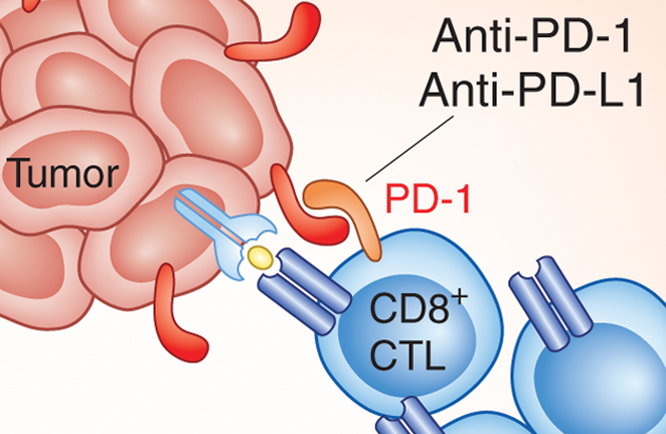
June 17, 2015 – Bristol Myers Squipp recently announced that the U.S. Food and Drug Administration (FDA) has approved Nivolumab (Opdivo) injection, for intravenous use, for the treatment of patients with metastatic squamous non-small cell lung cancer (NSCLC) with progression on or after platinum-based chemotherapy. Nivolumab (Opdivo) is the first PD-1 (programmed death receptor-1) therapy to demonstrate overall survival in previously treated metastatic squamous NSCLC. Nivolumab (Opdivo) demonstrated significantly superior overall survival (OS) vs. docetaxel, with a 41% reduction in the risk of death (hazard ratio: 0.59 [95% CI: 0.44, 0.79; p=0.00025]), in a prespecified interim analysis of a Phase III clinical trial. The median OS was 9.2 months in the Opdivo arm (95% CI: 7.3, 13.3) and 6 months in the docetaxel arm (95% CI: 5.1, 7.3).
 In this context, CheckMate -017 was a landmark Phase III, open-label, randomized, multinational, multicenter clinical trial that evaluated Nivolumab (Opdivo) (3 mg/kg intravenously over 60 minutes every two weeks) (n=135) vs. standard of care, docetaxel (75 mg/m2 intravenously administered every 3 weeks) (n=137), in patients with metastatic squamous NSCLC who had progressed during or after prior platinum doublet-based chemotherapy regimen. This trial included patients regardless of their PD-L1 (programmed death ligand-1) status. The primary endpoint of this trial was overall survival (OS). The trial was stopped based on an assessment conducted by the independent Data Monitoring Committee (DMC), which concluded that the study met its endpoint, demonstrating superior OS in patients receiving Nivolumab (Opdivo) compared to docetaxel. The prespecified interim analysis was conducted when 199 events (86% of the planned number of events for final analysis) were observed (86 in the Opdivo arm and 113 in the docetaxel arm).
In this context, CheckMate -017 was a landmark Phase III, open-label, randomized, multinational, multicenter clinical trial that evaluated Nivolumab (Opdivo) (3 mg/kg intravenously over 60 minutes every two weeks) (n=135) vs. standard of care, docetaxel (75 mg/m2 intravenously administered every 3 weeks) (n=137), in patients with metastatic squamous NSCLC who had progressed during or after prior platinum doublet-based chemotherapy regimen. This trial included patients regardless of their PD-L1 (programmed death ligand-1) status. The primary endpoint of this trial was overall survival (OS). The trial was stopped based on an assessment conducted by the independent Data Monitoring Committee (DMC), which concluded that the study met its endpoint, demonstrating superior OS in patients receiving Nivolumab (Opdivo) compared to docetaxel. The prespecified interim analysis was conducted when 199 events (86% of the planned number of events for final analysis) were observed (86 in the Opdivo arm and 113 in the docetaxel arm).
Currently, Nivolumab (Opdivo) is the only FDA-approved monotherapy to demonstrate proven superior OS compared to standard of care in more than 15 years in previously treated metastatic squamous NSCLC. “The FDA approval of Nivolumab (Opdivo)introduces an entirely new treatment modality that has demonstrated unprecedented results for the treatment of previously treated metastatic squamous NSCLC, with the potential to replace chemotherapy for these patients,” said Dr. Suresh Ramalingam, MD, Professor and Director of Medical Oncology, Winship Cancer Institute of Emory University. “This milestone brings to fruition the long-held hope that immuno-oncology medicines can be significantly effective in this difficult-to-treat population.”
Therapy of eligible patients with Nivolumab (Opdivo) comes with a price, however. The clinical safety profile of Nivolumab (Opdivo) in squamous NSCLC was established in CheckMate -063, a Phase II single-arm, open-label, multinational, multicenter trial of Nivolumab (Opdivo), administered as a single agent in patients with metastatic squamous NSCLC who have progressed after receiving a platinum-based therapy and at least one additional systemic treatment regimen (n=117). Patients received 3 mg/kg of Nivolumab (Opdivo) administered intravenously over 60 minutes every 2 weeks. This trial included patients regardless of their PD-L1 status. The most common adverse reactions (reported in ≥20% of patients) were fatigue (50%), dyspnea (38%), musculoskeletal pain (36%), decreased appetite (35%), cough (32%), nausea (29%), and constipation (24%). Serious adverse reactions occurred in 59% of patients receiving Nivolumab (Opdivo). The most frequent serious adverse reactions reported in ≥2% of patients were dyspnea, pneumonia, chronic obstructive pulmonary disease exacerbation, pneumonitis, hypercalcemia, pleural effusion, hemoptysis, and pain. Nivolumab (Opdivo)was discontinued due to adverse reactions in 27% of patients. Twenty-nine percent of patients receiving Nivolumab (Opdivo) had a drug delay for an adverse reaction.
With at least 10 months of minimum follow up for all patients, the confirmed objective response rate (ORR), the study’s primary endpoint, was 15% (17/117) (95% CI = 9, 22) of which all were partial responses. The median time to onset of response was 3.3 months (range: 1.7 to 8.8 months) after the start of Nivolumab (Opdivo)treatment. Seventy-six percent of Nivolumab (Opdivo) responders (13/17 patients) had ongoing responses with durability of response ranging from 1.9+ to 11.5+ months; 10 of these 17 (59%) patients had durable responses of 6 months or longer.
“The approval of Nivolumab (Opdivo) for the treatment of previously treated metastatic squamous non-small cell lung cancer is a major advancement in delivering extended survival for patients fighting this deadly disease,” said Andrea Ferris, President and Chairman, Lungevity Foundation. “We are very excited for an immuno-oncology therapy to enter the market and offer options and hope for many of our patients.
Previously, Nivolumab (Opdivo) was approved by the FDA for the tratment of advanced melanoma.


Asking questions are really pleasant thing if you are not understanding something fully, except this article gives nice understanding yet.
Great article.
Greate article. Keep writing such kind of information on your page.
Im really impressed by your site.
Hey there, You’ve performed a great job. I will definitely digg it and in my view suggest to my friends.
I’m confident they’ll be benefited from this web site.
Ridiculous story there. What happened after? Good luck!
Helpful info. Fortunate me I found your website unintentionally, and I’m stunned why this twist of
fate did not came about earlier! I bookmarked it.
This design is steller! You obviously know how to keep a reader amused.
Between your wit and your videos, I was almost moved to start my own blog (well, almost…HaHa!) Wonderful job.
I really loved what you had to say, and more than that, how you presented it.
Too cool!
Hello there, I discovered your site via Google even as looking for a comparable subject, your web site got here up, it appears
great. I have bookmarked it in my google bookmarks.
Hi there, just turned into alert to your blog via Google, and located that it’s really informative.
I’m gonna be careful for brussels. I’ll appreciate in the event you continue this in future.
A lot of folks shall be benefited out of your writing.
Cheers!
We absolutely love your blog and find most of your post’s to be precisely what I’m
looking for. Would you offer guest writers to write content for you?
I wouldn’t mind composing a post or elaborating on a few of the subjects you write
in relation to here. Again, awesome site!
Wonderful site. A lot of useful information here. I’m sending it to
several friends ans additionally sharing in delicious.
And obviously, thanks on your sweat!
Hello there! This post could not be written any better! Reading
this post reminds me of my previous room mate! He
always kept chatting about this. I will forward this page
to him. Fairly certain he will have a good read.
Thanks for sharing!
Hi there! Would you mind if I share your blog with my facebook group?
There’s a lot of people that I think would really appreciate your
content. Please let me know. Thanks
Hi, no, I would not mind. The more people know “thasso”,the better. The more people may eventually take some useful information from it, the better.
Simply want to say your article is as astonishing.
The clearness on your put up is just great and
i could think you’re knowledgeable on this subject.
Fine with your permission let me to take hold of your RSS feed to keep updated with imminent post.
Thanks a million and please carry on the gratifying work.
Thanks for finally writing about >Theragenomic medicine: Nivolumab (Opdivo) | thasso <Liked it!
Thank you for the auspicious writeup. It in fact was a amusement account it.
Look advanced to more added agreeable from you!
By the way, how could we communicate?
Asking questions are actually good thing if you
are not understanding something fully, except this paragraph provides pleasant
understanding yet.
I am actually thankful to the holder of this web site who has shared this wonderful
post at at this time.
It’s really a cool and helpful piece of info. I’m happy that you just shared this useful info with us. Please keep us up to date like this. Thank you for sharing.|
I pointed out that by the comments already here, you might be a leader in your industry
Audio started playing any time I opened this web page, so irritating!
Which audio started playing? There shouldn’t be any. Please specify.
Great beat ! I wish to apprentice while you amend your web site, how could i subscribe for a blog site? The account aided me a acceptable deal. I had been tiny bit acquainted of this your broadcast offered bright clear concept
6/22/2015: EU approves Bristol-Myers’ Opdivo for melanoma
We have just learned that the PD-1 inhibitor Opdivo, or nivolumab, has obtained approval from the European Commission as a treatment for unresectable or metastatic melanoma. The decision was backed by data from two trials showing the drug can improve response rate and overall survival, compared with standard care.
Hi drive, thank you for your kind comment. This is very encouraging indeed. As far as your questions go: Yes, I have choosen the paid version of the WordPress Theme “Responsive” which is called “Responsive Pro” for my blog and homepage. Simply because it also displays, with some restrictions, on tablets and smartphones. One day, I would love to implement a true App-Version for smartphones; however, my time and funds are so limited,it will take for ever to realize this dream. I keep trying.
I’m really impressed with your writing skills and also with the layout on your blog. Is this a paid theme or did you customize it yourself? Anyway keep up the excellent quality writing, it’s rare to see a nice blog like this one these days..
Bristol Myers Squibb, the maker of Opdivo, provides some more iinformation on the side effects of Opdivo. Please read on:
Important Safety Information for OPDIVO® (nivolumab)
OPDIVO® (nivolumab) is a medicine that may treat your melanoma or lung cancer by working with your immune system. OPDIVO can cause your immune system to attack normal organs and tissues in many areas of your body and can affect the way they work. These problems can sometimes become serious or life-threatening and can lead to death. These problems may happen anytime during treatment or even after your treatment has ended.
Serious side effects may include:
Lung problems (pneumonitis). Symptoms of pneumonitis may include: new or worsening cough; chest pain; and shortness of breath.
Intestinal problems (colitis) that can lead to tears or holes in your intestine. Signs and symptoms of colitis may include: diarrhea (loose stools) or more bowel movements than usual; blood in your stools or dark, tarry, sticky stools; and severe stomach area (abdomen) pain or tenderness.
Liver problems (hepatitis). Signs and symptoms of hepatitis may include: yellowing of your skin or the whites of your eyes; severe nausea or vomiting; pain on the right side of your stomach area (abdomen); drowsiness; dark urine (tea colored); bleeding or bruise more easily than normal; and feeling less hungry than usual.
Kidney problems, including nephritis and kidney failure. Signs of kidney problems may include: decrease in the amount of urine; blood in your urine; swelling in your ankles; and loss of appetite.
Hormone gland problems (especially the thyroid, pituitary, and glands). Signs and symptoms that your hormone glands are not working properly may include: headaches that will not go away or unusual headaches; extreme tiredness, weight gain or weight loss; changes in mood or behavior, such as decreased sex drive, irritability, or forgetfulness; dizziness or fainting; hair loss; feeling cold; constipation; and voice gets deeper.
Problems in other organs. Signs of these problems include: rash; changes in eyesight; severe or persistent muscle or joint pains; and severe muscle weakness.
Getting medical treatment right away may keep these problems from becoming more serious.
Your doctor will check you for these problems during treatment with OPDIVO. Your doctor may treat you with corticosteroid medicines and delay or completely stop treatment with OPDIVO, if you have severe side effects.
Pregnancy and Nursing:
Tell your healthcare provider if you are pregnant or plan to become pregnant. OPDIVO can harm your unborn baby. Females who are able to become pregnant should use an effective method of birth control during and for at least 5 months after the last dose of OPDIVO. Talk to your doctor about birth control methods that you can use during this time. Tell your doctor right away if you become pregnant during treatment with OPDIVO. Before receiving OPDIVO, tell your healthcare provider if you are breastfeeding or plan to breastfeed. It is not known if OPDIVO passes into your breast milk. Do not breastfeed during treatment with OPDIVO.
Tell your healthcare provider about:
Your health problems or concerns if you: have immune system problems such as Crohn’s disease, ulcerative colitis, or lupus; have had an organ transplant; have lung, or breathing problems; have liver problems; or have any other medical conditions
All the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements
The most common side effects of OPDIVO in people with melanoma include: rash.
The most common side effects of OPDIVO in people with squamous non-small cell lung cancer include: feeling tired; shortness of breath; pain in muscles, bones, and joints; decreased appetite; cough; nausea; and constipation.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of OPDIVO. For more information, ask your healthcare provider or pharmacist.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit http://www.fda.gov/medwatch or call 1-800-FDA-1088.