Cobimetinib (Cotellic) is now approved as part of a combination treatment with Vemurafenib (Zelboraf) for advanced melanoma

Last Updated on November 13, 2015 by Joseph Gut – thasso
November 13, 2015 – The American Food and Drug Administration (FDA) just approved Cobimetinib (Cotellic) to be used in combination with Vemurafenib (Zelboraf) to treat advanced melanoma that has spread to other parts of the body or can’t be removed by surgery, and that has a certain type of abnormal gene (BRAF V600E or V600K mutation). Already in September 2015, the European Medicines Agency (EMA) issued a positive opinion for approval of Cobimetinib (Cotellic) for the same indication.
Melanoma is the most aggressive and dangerous form of skin cancer. It forms in the skin cells that develop the skin’s pigment and if not diagnosed early, the cancer
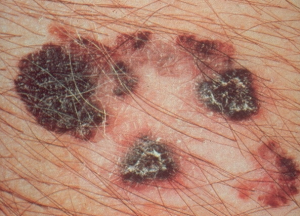
is likely to spread to other parts of the body. Cobimetinib (Cotellic) works by blocking the activity of an enzyme known as MEK, which is part of a larger signaling pathway (the Ras-Raf-MEK-ERK pathway). Abnormal activity of signaling pathways can lead to cancer. Cobimetinib (Cotellic) prevents or slows cancer cell growth. Vemurafenib (Zelboraf) in itself is a BRAF inhibitor that affects a different part of the same pathway and was approved in the US in 2011 to treat patients with melanoma that has spread to other parts of the body or cannot be removed by surgery, whose tumors express a gene mutation called BRAF V600E, as detected by an FDA approved test. Health care providers should confirm the presence of BRAF V600E or BRAF V600K mutation in their patients’ tumor specimens using one of the available FDA approved tests prior to starting treatment with Cobimetinib (Cotellic) in combination with Vemurafenib (Zelboraf).
As clinical basis for the FDA approval, the safety and efficacy of Cobimetinib (Cotellic) taken in combination with Vemurafenib (Zelboraf) were demonstrated in a randomized clinical study of 495 patients with previously untreated, BRAF V600 mutation-positive melanoma that is advanced or cannot be removed by surgery. All study participants received Vemurafenib (Zelboraf) and were then randomly selected to also take either Cobimetinib (Cotellic) or a placebo. On average, patients taking Cobimetinib (Cotellic) plus Vemurafenib (Zelboraf) experienced a delay in the amount of time it took for their disease to worsen (approximately 12.3 months after starting treatment) compared to approximately 7.2 months after starting treatment for those taking Vemurafenib (Zelboraf) only. In addition, patients taking Cobimetinib (Cotellic) plus Vemurafenib (Zelboraf) lived longer, with approximately 65 percent of patients alive 17 months after starting treatment as compared to half of those taking vemurafenib only. Additionally, 70 percent of those taking Cobimetinib (Cotellic) plus Vemurafenib (Zelboraf) experienced complete or partial shrinkage of their tumors, compared to 50 percent among those taking Vemurafenib (Zelboraf) plus placebo.
Cobimetinib (Cotellic) may cause severe adverse effects including damage to the heart muscle (cardiomyopathy) or to other muscles (rhabdomyolysis), new skin tumors (primary cutaneous malignancies), eye disease (retinal detachment), severe skin rash, liver damage (hepatotoxicity), hemorrhage and severe skin rash due to increased sensitivity to sunlight (photosensitivity). People taking Cobimetinib (Cotellic) should avoid sun exposure, wear protective clothing, and a broad spectrum ultraviolet A/ultraviolet B sunscreen to protect against sunburn. Women taking Cobimetinib (Cotellic) should use effective contraception, as the medication can cause harm to a developing fetes. Other than that, the most common side effects of treatment with Cobimetinib (Cotellic) in combination with Vemurafenib (Zelboraf) are diarrhea, nausea, fever (pyrexia) and vomiting.
See here an early commentary on the therapy of Cobimetinib (Cotellic) in combination with Vemurafenib (Zelboraf):
For Vemurafenib (Zelboraf) see also two earlier posts on Thasso Post here and here. Particularly the second article addressed the question of secondary tumors being associated with Vemurafenib (Zelboraf) therapy. As we have learned above, the occurrence of new skin tumors (primary cutaneous malignancies) is still a problem also with Cobimetinib (Cotellic) in combination with Vemurafenib (Zelboraf).

