The first gene therapy FDA approved in the US

Last Updated on October 23, 2017 by Joseph Gut – thasso
Overall, with Tisagenlecleucel (Kymriah) a new frontier in medical innovation with the ability to reprogram a patient’s own cells to attack a deadly cancer has been entered. New technologies such as gene and cell therapies hold out the potential to transform medicine and create an inflection point in the ability to treat and even cure many intractable illnesses. Tisagenlecleucel (Kymriah) is a first-of-its-kind treatment approach that fills an important unmet need for children and young adults with B-cell ALL. Not only does Tisagenlecleucel (Kymriah) provide these patients with a new treatment option where very limited options exist, but a treatment option that has shown promising remission and survival rates in clinical trials. The efficacy of Tisagenlecleucel (Kymriah) was demonstrated in one multicenter clinical trial of 63 pediatric and young adult patients with relapsed or refractory B-cell precursor ALL. The overall remission rate within three months of treatment was 83 percent.
The flip side of the coin certainly is that the treatment with Tisagenlecleucel (Kymriah) has the potential to cause severe side effects. Tisagenlecleucel (Kymriah) carries a Boxed Warning for cytokine release syndrome (CRS), which is a systemic response to the activation and proliferation of CAR T-cells causing high fever and flu-like symptoms, and for neurological events. Both CRS and neurological events can be life-threatening. Other severe side effects of Tisagenlecleucel (Kymriah) include serious infections, low blood pressure (hypotension), acute kidney injury, fever, and decreased oxygen (hypoxia). Most symptoms appear within one to 22 days following infusion of Tisagenlecleucel (Kymriah). Since the CD19 antigen is also present on normal B-cells, and Tisagenlecleucel (Kymriah) will also destroy those normal B cells that produce antibodies, there may be an increased risk of infections for a prolonged period of time.
The FDA today also expanded the approval of Tocilizumab (Actemra) to treat CAR T-cell-induced severe or life-threatening CRS in patients 2 years of age or older. In clinical trials in patients treated with CAR-T cells, 69 percent of patients had complete resolution of CRS within two weeks following one or two doses of Tocilizumab (Actemra).
Because of the very serious risks for CRS and neurological events, Tisagenlecleucel (Kymriah) is being approved with a risk evaluation and mitigation strategy (REMS), which includes elements to assure safe use (ETASU). The FDA is requiring that hospitals and their associated clinics that dispense Tisagenlecleucel (Kymriah) be specially certified. As part of that certification, staff involved in the prescribing, dispensing, or administering of Tisagenlecleucel (Kymriah) are required to be trained to recognize and manage CRS and neurological events. Additionally, the certified health care settings are required to have protocols in place to ensure that Tisagenlecleucel (Kymriah) is only given to patients after verifying that tocilizumab is available for immediate administration. The REMS program specifies that patients be informed of the signs and symptoms of CRS and neurological toxicities following infusion, and of the importance of promptly returning to the treatment site if they develop fever or other adverse reactions after receiving treatment with Tisagenlecleucel (Kymriah).
To further evaluate the long-term safety, the Marketing Authorisation Holder (Novartis Pharmaceuticals Corp) is also required to conduct a post-marketing observational study involving patients treated with Tisagenlecleucel (Kymriah).
Related Information
- FDA: Cellular and Gene Therapy Products
- FDA: Oncology Center of Excellence
- NIH: Childhood Acute Lymphoblastic Leukemia
- FDA: What is Gene Therapy?
- FDA: Kymriah (tisagenlecleucel) product page
- FDA: Approved Cellular and Gene Therapy Products
See an introductory video on gene therapy here:
________________________

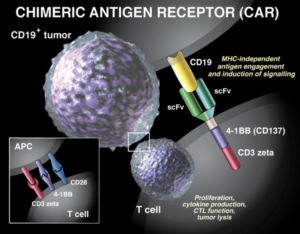 Tisagenlecleucel (Kymriah) is a genetically-modified autologous T-cell immunotherapy. Each dose of Tisagenlecleucel (Kymriah) is a customized treatment created using an individual patient’s own T-cells, a type of white blood cell known as a lymphocyte. The patient’s T-cells are collected and sent to a manufacturing center where they are genetically modified to include a new gene that contains a specific protein (a chimeric antigen receptor (CAR)) that directs the T-cells to target and kill leukemia cells that have the CD19 specific antigen (
Tisagenlecleucel (Kymriah) is a genetically-modified autologous T-cell immunotherapy. Each dose of Tisagenlecleucel (Kymriah) is a customized treatment created using an individual patient’s own T-cells, a type of white blood cell known as a lymphocyte. The patient’s T-cells are collected and sent to a manufacturing center where they are genetically modified to include a new gene that contains a specific protein (a chimeric antigen receptor (CAR)) that directs the T-cells to target and kill leukemia cells that have the CD19 specific antigen (





Chimeric antigen receptor (CAR) T-cell therapy has emerged as a breakthrough in the treatment of leukemia and lymphoma, but it has been associated with unique acute toxicities that are uncommon with other cancer therapies.
There have been published recommendations to handle at least two of the specific toxicities unique to treatment with these agents: cytokine-release syndrome (CRS) and CAR T-cell–related encephalopathy syndrome (CRES).
See more here: http://www.medscape.com/viewarticle/886005?src=wnl_edit_tpal&uac=190401EN
See here the published article: https://www.nature.com/articles/nrclinonc.2017.148.epdf?referrer_access_token=Ia1V8rXg4jr7G017hNxeS9RgN0jAjWel9jnR3ZoTv0P_TtKy7kWlBFD4lKDglGt7Y75o38zJSee_dkc9pPHUDRllxmR5ENvdTon0pr6MQsLJJq7ZowkntWdW6Q9-w9_6x8AR0a6L_F5cYqa7gKBx4wm1EwFkA4bMEd2xnACtJ9LvXAoCugKa0nUDIUc4VHxNy1smLvwTVTns40vGt1Z7JeJpxeSrMiBsO1i9p6rqXVt5nZaj5ge4uOgJgar-haPI&tracking_referrer=www.medscape.com
Earlier, not yet FDA-approved gene therapy approaches, particularly those for treating SCID in children were met with limited success: Some children developed leukemias, particularly when treated for SCID-X, while those treated for ADA-SCID did seemingly not develop the condition. There resulted a hot debate about the actual strategic approaches and the safety of gene therapy. It will be interesting to see how CAR T-Cell therapy will perform in the long term.
It may be noteworthy that the European Commission approved the gene therapy Strimvelis on May 27, 2016, to treat children with ADA-SCID which is due to adenosine deaminase deficiency.
See here: http://www.medscape.com/viewarticle/861416