Risk of severe if not fatal skin reactions with Pembrolizumab (Keytruda)
Last Updated on April 19, 2017 by Joseph Gut – thasso
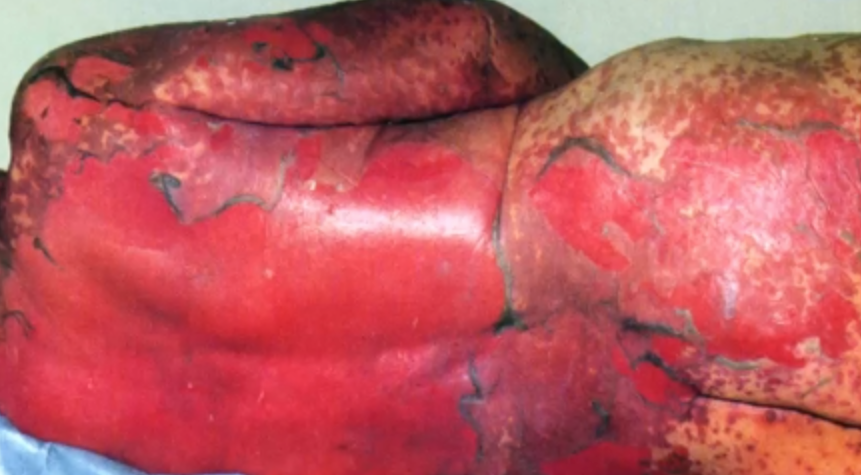
March 21, 2017 – Health Canada just communicated this important safety information: Cases of Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) under treatment of patients with Pembrolizumab (Keytruda) have been reported internationally in the clinical trials and post-marketing setting. There were 8 cases of SJS (1 of them with a fatal outcome) and 2 cases of TEN (1 of them with a fatal outcome). Of these reports, one case of SJS was reported in Canada.

In Canada, Pembrolizumab (Keytruda), a programmed death receptor-1 (PD-1) blocking antibody (a so-called checkpoint inhibitor), is indicated for the treatment of patients with unresectable or metastatic melanoma who have not received prior treatment with Ipilimumab (Yervoy). Pembrolizumab (Keytruda) is also authorized under the notice of compliance with conditions (NOC/c) policy for the treatment of patients with i) unresectable or metastatic melanoma and disease progression following Ipilimumab (Yervoy) therapy and, if BRAF V600 mutation positive, following a BRAF or MEK inhibitor, and ii) metastatic non-small cell lung carcinoma (NSCLC) whose tumours express PD-L1 (as determined by a validated test) and who have disease progression on or after platinum-containing chemotherapy.
The potential risk of SJS and TEN with Pembrolizumab (Keytruda) use was evaluated using currently available safety data from the published literature and the manufacturer’s global safety database (which includes both clinical trial serious adverse events and post-marketing reports). One fatal case of SJS in a clinical trial and one fatal case of TEN in the post-marketing setting have been reported internationally in patients treated with Pembrolizumab (Keytruda). Including these cases, there have been 8 cases of SJS (6 in clinical trials, and 2 post-marketing) and 2 of TEN (both post-marketing). Of these 10 reports, one case of SJS was reported in Canada. Two of the 10 reports had a temporal association with Pembrolizumab (Keytruda) use, had few confounding factors, and had skin biopsies that were consistent with SJS or TEN. These cases come out of approximately 11,000 patients in clinical trials and 27,000 patients in the post-marketing setting who have been treated with Pembrolizumab (Keytruda).
Healthcare professionals including oncologists, nurses, hospital pharmacists, emergency room staff, and cancer clinic staff should i) counsel patients about the benefits and risks of Pembrolizumab (Keytruda), including the risk and early symptoms of SJS and TEN, ii) suspend Pembrolizumab (Keytruda) treatment and refer for immediate specialized evaluation and treatment if a patient reports any severe skin reaction, or in a case of suspected SJS or TEN, and iii) permanently discontinue Pembrolizumab (Keytruda) if SJS or TEN is confirmed.
Patients should inform their healthcare professional if they are experiencing a side effect related to Pembrolizumab (Keytruda) use, and both, healthcare professionals and patients alike may report any case of severe skin reactions or suspected SJS or TEN or any other serious or unexpected side effects when receiving Pembrolizumab (Keytruda) to Merck Canada Inc. or Health Canada since managing marketed health product-related side effects largely depends on these contributions.
These are the addresses:
Merck Canada Inc., Pharmacovigilance
16750 Trans -Canada Hwy.
Kirkland, Québec, H9H 4M7
Fax.: 1-800-3693090
Marketed Health Products Directorate
E-mail: mhpd_dpsc@hc-sc.gc.ca
Telephone: 613-964-6522
Fax: 613-952-7738
You can report any suspected adverse reactions associated with the use of health products to Health Canada by i) calling toll-free at 1-866-234-2345, or by ii) Visiting MedEffect Canada’s Web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax.
Outside of Canada, healthcare professionals and patients alike may report any case of severe skin reactions or suspected SJS or TEN or any other serious or unexpected side effects when receiving Pembrolizumab (Keytruda) to their country specific Merck (or Merck, Sharp & Dohme (MSD) where it applies) Pharmacovigilance Center or to the county specific registration authority’s Pharmacovigilance Center.






