Rare cases of anaphylaxis with COVID-19 vaccines
Last Updated on January 26, 2021 by Joseph Gut – thasso
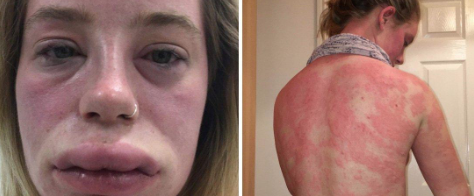
January 26, 2021 – To date, a rare serious adverse (drug) reaction (SADR) has been noted to be associated with the first two Covid-19 vaccines, i.e., the one from Pfizer-BioNTech and the one from Moderna. We are talking about anaphylaxis, which is a severe allergic reaction of rapid onset and which may be fatal if not treated immediately.
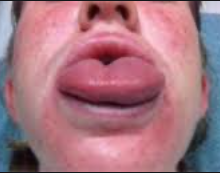 Anaphylaxis typically causes either an itchy rash, throat or tongue swelling, shortness of breath, vomiting, lightheadedness, or low blood pressure. Often, a combination of these symptoms may occur at the same time. Overall, anaphylaxis constitutes a serious medical emergency that may require resuscitation measures such as airway management, supplemental oxygen, large volumes of intravenous fluids, and close monitoring. Immediate administration of epinephrine (adrenaline) is the treatment of choice with antihistamines and steroids (for example, dexamethasone) often used as adjuncts. A period of in-hospital observation for between 2 and 24 hours is recommended for people once they have returned to normal due to concerns of biphasic course of anaphylaxis.
Anaphylaxis typically causes either an itchy rash, throat or tongue swelling, shortness of breath, vomiting, lightheadedness, or low blood pressure. Often, a combination of these symptoms may occur at the same time. Overall, anaphylaxis constitutes a serious medical emergency that may require resuscitation measures such as airway management, supplemental oxygen, large volumes of intravenous fluids, and close monitoring. Immediate administration of epinephrine (adrenaline) is the treatment of choice with antihistamines and steroids (for example, dexamethasone) often used as adjuncts. A period of in-hospital observation for between 2 and 24 hours is recommended for people once they have returned to normal due to concerns of biphasic course of anaphylaxis.
Apparently, until mid January 2021 environ, there have been about 11.1 cases of anaphylaxis per million doses with the Pfizer-BioNTech COVID-19 vaccine (on the market under the name Comirnaty in some parts of the World, notably in Europe) in the US, which is higher than the estimated 1.3 cases per million doses  with influenza vaccines. Anaphylaxis has been noted as an SARD with both, the Pfizer-BioNTech Covid-19 vaccine and the Moderna Covid-19 vaccine. The Food and Drug Administration (FDA) granted emergency use authorizations (EUA) for these two vaccines in December 2020; meanwhile, the two products have received conditional approvals in the European Union (by EMA) and full authorisation by several additional health authorities as well.
with influenza vaccines. Anaphylaxis has been noted as an SARD with both, the Pfizer-BioNTech Covid-19 vaccine and the Moderna Covid-19 vaccine. The Food and Drug Administration (FDA) granted emergency use authorizations (EUA) for these two vaccines in December 2020; meanwhile, the two products have received conditional approvals in the European Union (by EMA) and full authorisation by several additional health authorities as well.
At least based on data from the US, even though anaphylaxis is a very serious adverse reaction and with the cases seen to date, the COVID-19 vaccines remain a good value proposition. The very rare occurrence of anaphylaxis must be balanced against the threat of COVID-19, which has and still does claim about 2000 lives a day in the US, with similar proportions of the populace affected in other countries worldwide.
However, it is absolutely mandatory that every healthcare provider is ready to treat rare cases of anaphylaxis following administration of COVID-19 vaccines. That means that minimally, vaccinating health care providers do not only have an EpiPen available and ready, but also know how to us it in case a patient needs an immediate anti-anaphylaxis intervention in case of urgency. In order to further minimise the risk for occurrence of anaphylaxis, health care providers should ask their patients before vaccination about the patients possible known tendency for developing allergies of all causes. Since there is increasing genetic evidence for personal predispositions and that least some allergies might run in families, knowing about allergies of family member might also help the physician to estimate the risk for an anaphylaxis occurring and let him (the physician) at least take the precautionary measures.
For further informations concerning the safety of Covid-19 vaccines, you may follow the Morbidity and Mortality Weekly Reports (MMWR) by the US Centers of Disease Control.
See here a short sequence about the concerns about anaphylaxis associated with Covid-19 vaccination:

