Obeticholic Acid (Ocaliva): Is there a serious drug safety problem?
Last Updated on February 8, 2018 by Joseph Gut – thasso
September 30, 2017 – Obeticholic acid (Ocaliva) was approved by the American Food & Drug Administration (FDA) in May 2016 for the treatment of primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA) in adult patients with an inadequate response to UDCA, or as monotherapy in adults unable to tolerate UDCA.
On September 21, 2017, the FDA has issued a Drug Safety Communication warning about about serious if not fatal liver injury in some patients taking Obeticholic acid (Ocaliva) for treatment of PBC. PBC causes the bile ducts in the liver to become inflamed, damaged and destroyed. This causes bile to build up in the liver. This build-up damages the liver over time, eventually causing it to lose its ability to function properly. Obeticholic acid (Ocaliva) has been shown to improve the condition.
In the 13 months after Obeticholic acid (Ocaliva) was approved in May 2016, FDA received, according to their newest Drug Safety Communication, reports of serious liver injury or death associated with Obeticholic acid (Ocaliva). The FDA’s Adverse Event Reporting System (FAERS) includes only reports submitted to FDA, so there may be unknown figure of unreported additional cases about which FDA are unaware.
Nineteen cases of death were identified, of which eight provided information about the patient’s cause of death. The cause of death was reported to be worsening of PBC disease in seven cases, with cardiovascular disease cited in the other case. Seven of these eight cases described patients with moderate to severe decreased liver function who received Obeticholic acid (Ocaliva) 5 mg daily, instead of a dose no greater than 10 mg twice weekly as recommended in the label prescribing information for patients with this extent of decreased liver function.
Additionally, FDA also identified 11 cases of serious liver injury with Obeticholic acid (Ocaliva) use. Six of the patients who had moderate or severe decreases in liver function at baseline and developed serious liver injury were receiving Obeticholic acid (Ocaliva) 5 mg daily, instead of a dose no greater than 10 mg twice weekly as recommended by FDA in the drug label. Three of these six patients died, which were included in the 19 death cases mentioned previously. Obeticholic acid (Ocaliva) was discontinued in four of six cases, which resulted in one patient experiencing symptom resolution and an improvement in a liver blood test. The remaining three cases did not report the response after discontinuation. The other five cases of serious liver injury were reported in patients with no or mild decreases in liver function prior to initiating Obeticholic acid (Ocaliva). Four of these five patients received Ocaliva 5 mg daily, and one did not report the dose. Obeticholic acid (Ocaliva) was discontinued in all five cases, which resulted in one patient experiencing symptom resolution and one patient experiencing improved liver blood tests and symptom resolution. The remaining three cases did not report the response after discontinuation.
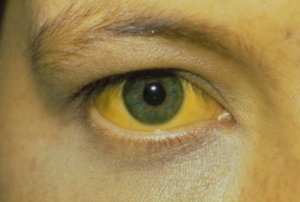
FDA recommends that patients should contact their health care professional if they have questions or concerns about taking Obeticholic acid (Ocaliva). They should report new or worsening severe skin itching to their health care professional. They should also contact them immediately if patients develop any of the following symptoms that may be signs of liver injury: yellow eyes or skin, new or worsening fatigue, diarrhea, weight loss, abdominal pain, decreased appetite, nausea and vomiting, change in behavior or confusion, vague symptoms such as anxiety or unease,abdominal swelling, and bloody stools.
For health care professionals, FDA recommends to determine the patient’s baseline liver function prior to starting Obeticholic acid (Ocaliva). Patients with moderate to severe liver impairment (Child-Pugh B and C) should be started on the approved dosing schedule of 5 mg once weekly, rather than the 5 mg daily dosing used for other PBC patients, and if needed, can be increased up to a maximum approved dose of 10 mg twice weekly. Health care professionals should monitor patients frequently for disease progression, and reduce the dosing frequency to once- or twice-weekly for patients who progress to moderate or severe liver impairment. In all patients treated with Obeticholic acid (Ocaliva), monitor frequently for liver injury (e.g., worsened liver blood tests and adverse liver-related reactions that may be inconsistent with the patient’s extent of disease). If liver injury is suspected, discontinue Obeticholic acid (Ocaliva).
Given the extensive instructions on dosing, the question arises here, if this medication is too difficult to handle in general practice. The question arises if the addition of a Boxed Warning to the drug label would further alert healthcare providers and patients alike for the presumed dosing difficulties associated with Obeticholic acid (Ocaliva). Furthermore, given the significant number of very serious and fatal cases that have occurred in patients taking Obeticholic acid (Ocaliva), one might ask the question if FDA should not halt Obeticholic acid (Ocaliva) and at least temporarily withdraw from the market until the etiology behind the reported serious and fatal event after Obeticholic acid (Ocaliva) are better understood and become amenable to genuinely avoiding, in order to prevent further fatal patient harm to happen.
_____________







On February 1, 2018 the FDA issued a Safety Alert, entitled “Ocaliva (obeticholic acid): Drug Safety Communication – Boxed Warning Added To Highlight Correct Dosing”.
The Boxed Warning says:
__________________________
WARNING: HEPATIC DECOMPENSATION AND FAILURE IN
INCORRECTLY DOSED PBC PATIENTS WITH CHILD-PUGH
CLASS B OR C OR DECOMPENSATED CIRRHOSIS
See full prescribing information for complete boxed warning
• In postmarketing reports, hepatic decompensation and failure, in
some cases fatal, have been reported in patients with primary biliary
cholangitis (PBC) with decompensated cirrhosis or Child-Pugh Class
B or C hepatic impairment when OCALIVA was dosed more
frequently than recommended. (5.1)
• The recommended starting dosage of OCALIVA is 5 mg once weekly
for patients with Child-Pugh Class B or C hepatic impairment or a
prior decompensation event. (2.2)
_________________________
See the latest drug label for Ocaliva here:
https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm594901.htm
See the Safety Alert by the FDA here:
https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm594901.htm