Daclizumab (Zinbryta) approved for the treatment of multiple sclerosis (MS)
Last Updated on May 28, 2016 by Joseph Gut – thasso
May 28, 2016 – Multiple sclerosis (MS) is a chronic, inflammatory, autoimmune disease of the central nervous system that disrupts communication between the brain and other parts of the body. It is among the most common causes of neurological disability in young adults and occurs more frequently in women than men. For most people with MS, episodes of worsening function (relapses) are initially followed by recovery periods (remissions). Over time, recovery may be incomplete, leading to progressive decline in function and increased disability. Most people experience their first symptoms of MS between the ages of 20 and 40.
The American Food & Drug Administration (FDA) has just added an additional treatment option to the armamentarium of drugs to fight MS by approving Daclizumab (Zinbryta) for the treatment of adults with relapsing forms of MS. Daclizumab (Zinbryta) is a long-acting injection that is self-administered by the
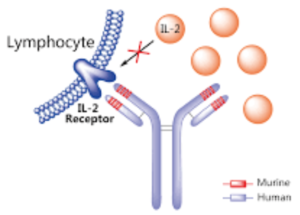
patient monthly. Daclizumab (Zinbryta) is a therapeutic humanized monoclonal antibody and works by binding to CD25, the alpha subunit of the IL-2 receptor of T-cells. Previously, Daclizumab was already FDA-approved under the trade name Zenapax to prevent rejection in organ transplantation, especially in kidney transplants.
According to FDA, the effectiveness of Zinbryta was shown in two clinical trials. One trial compared Daclizumab (Zinbryta) and Avonex in 1,841 participants who were studied for 144 weeks. Patients on Daclizumab (Zinbryta) had fewer clinical relapses than patients taking Avonex. The second trial compared Daclizumab (Zinbryta) with placebo and included 412 participants who were treated for 52 weeks. In that study, patients receiving Daclizumab (Zinbryta) had fewer relapses compared to those receiving placebo.
Daclizumab (Zinbryta) comes with serious safety risks, however, and should therefore generally be used only in patients who have had an inadequate response to two or more MS drugs previously. The most serious and sometimes fatal safety risks of Daclizumab (Zinbryta) include liver injury and immune conditions. Because of these risks, Daclizumab (Zinbryta) has a boxed warning and is available only through a restricted distribution program under a Risk Evaluation and Mitigation Strategy (REMS). The boxed warning tells prescribers that the drug can cause severe liver injury, including life-threatening and fatal events. Health care professionals should perform blood tests to monitor the patient’s liver function prior to starting Daclizumab (Zinbryta), monthly before each dose, and for up to six months after the last dose. The boxed warning also highlights other important risks of Zinbryta treatment including immune conditions, such as inflammation of the colon (non-infectious colitis), skin reactions, and enlargement of lymph nodes (lymphadenopathy). Additional highlighted warnings include hypersensitivity reactions (anaphylaxis or angioedema), increased risk of infections, and symptoms of depression and/or suicidal ideation.
The most common adverse reactions reported by patients receiving Daclizumab (Zinbryta) in the clinical trial that compared it to Avonex include cold symptoms (nasopharyngitis), upper respiratory tract infection, rash, influenza, dermatitis, throat (oropharyngeal) pain, eczema, and enlargement of lymph nodes. The most common adverse reactions reported by patients receiving Daclizumab (Zinbryta) when compared to placebo are depression, rash, and increased alanine aminotransferase, a fluid biomarker for disturbed liver function.


The European Medicines Agency has suspended the licence of Daclizumab (Zinbryta) a multiple sclerosis drug after 12 reports of serious inflammatory brain disorders, three of them fatal.
Daclizumab (Zinbryta) had already been withdrawn voluntarily worldwide by its manufacturers, Biogen and Abbvie because of the complexity of the adverse events being associated with the drug and the drugs benefits no longer outweighed its risks.
See here the final announcement by the EMA:
http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2018/05/news_detail_002955.jsp&mid=WC0b01ac058004d5c1