Cholangiocarcinoma: Diabetes Drugs In DPP-4 Inhibitor Class Associated With Doubled Risk
Last Updated on December 20, 2018 by Joseph Gut – thasso
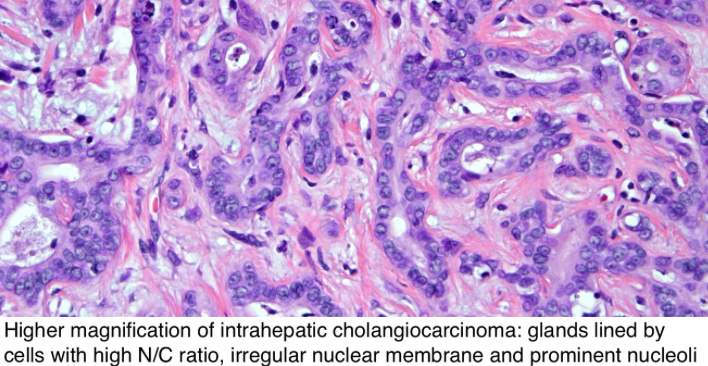
A very recent medical study published in the Britisch Medical Journal (BMJ) found that the use of dipeptidyl peptidase-4 (DPP-4) inhibitors was associated with a near doubling of the risk for cholangiocarcinoma. Similarly, the use of glucagon-like peptide-1 (GLP-1) receptor agonists was also associated with an increased hazard ratio of similar magnitude but generated a wide confidence interval that included even the null value, therefore rendering the finding with GLP-1 receptor agonist drugs less clear.
However, an association between incretin-based drugs and incidence of cholangiocarcinoma is biologically plausible. One mechanism could involve the increased GLP-1 levels associated with use of both DPP-4 inhibitors and GLP-1 receptor agonists; GLP-1 might promote the development of cholangiocarcinoma through its proliferative and anti-apoptotic effects on cholangiocytes. Another mechanism could involve chronic inflammation of the biliary epithelium, bile stasis, and bacterial infections, which might be of particular concern. These drugs have been associated with an increased risk of gallbladder related events (such as cholelithiasis, cholecystitis, cholangitis). Increased hazard ratios in secondary analyses assessing possible duration-response relations with DPP-4 inhibitors were noted. Specifically, the hazard ratio was particularly increased with cumulative durations ranging between one and two years of use and with more than two years since treatment initiation. Although these relatively rapid effects suggest that these drugs might act as tumour promoters among susceptible people, these secondary analyses were based on few events that generated wide confidence intervals and should thus be interpreted with caution.
To identify susceptible people (patients) it might well be worthwhile to beginn to look after the genetic profiles of people who preferentially develop cholangiocarcinoma when under DPP-4 inhibitor or GLP-1 receptor agonist therapy. What kind of genetic variations (allelic variants) of the DPP4 gene do these people carry? Similarly, what kind of variation of the GLP1R gene do they carry? May carrying particular allelic variants of the two genes (compound heterozygotes, extended on two genes, for example) render patients particularly and highly susceptible to the drug-induced development of this still very fatal type of cancer? By interaction of the genes? For the protection of patients from rare, yet fatal adverse effects, these are the king of questions that should be addressed in the age of theragenomic and personalized medicine and particularly in the field of individualized drug safety. It all turns around genetic susceptibility.
Here are the anti-diabetes drugs in the DPP-4 inhibitors class we just have been talking about:
- Januvia (Sitagliptin)
- Janumet (Sitagliptin / Metformin)
- Onglyza (Saxagliptin)
- Kombiglyze XR (Saxagliptin / Metformin)
- Qtern (Dapagliflozin / Saxagliptin)
- Nesina (Alogliptin)
- Kazano (Alogliptin / Metformin)
- Oseni (Alogliptin / Pioglitazone)
- Tradjenta (Linagliptin)
- Jentadueto (Linagliptin / Metformin)
- Glyxambi (Empagliflozin / Linagliptin)
The marketing names may vary in certain parts of the world; therefore, patients may want to refer to the names of the pharmacologically active ingredients (in brackets in the list of products above) in order to know if they take one of those products associated with the increased risk of development of cholangiocarcinoma. If so, please talk seriously to your treating physician.
See here just one of many videos on cholangiocarcinoma to give you some sort of impression on the seriousness of the condition, addressing mostly surgery in this case:

