Breast Cancer: Performance of prognostic signatures

Last Updated on February 17, 2018 by Joseph Gut – thasso
February 17, 2018 – In a new study, published in JAMA Oncology online on February 15, 2018, a comparison of the performance of 6 prognostic signatures for estrogen receptor (ER) –positive breast cancer was performed in a secondary analysis of a randomized clinical trial.
Almost all women with estrogen receptor (ER)–positive primary breast cancer are offered adjuvant endocrine therapy, and a highly relevant clinical question is who remains at high risk for distant recurrence despite completion of primary adjuvant therapy. Multigene expression profiles 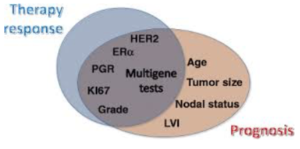 have significantly increased the ability to estimate distant recurrence in ER-positive breast cancer after surgery and endocrine treatment. These signatures are used in combination with different clinical characteristics to aid the selection of patients for whom chemotherapy may be appropriate based on prognosis. Several of these signatures are commercially available, including the Oncotype Dx recurrence score (RS) (Genomic Health), PAM50-based Prosigna risk of recurrence (ROR) (NanoString), Breast Cancer Index (BCI) (bioTheranostics), EndoPredict (EPclin) (Myriad Genetics), and MammaPrint Netherland Kanker Institute 70-gene signature (Agendia BV), are endorsed by several guidelines and are routinely used by clinicians.
have significantly increased the ability to estimate distant recurrence in ER-positive breast cancer after surgery and endocrine treatment. These signatures are used in combination with different clinical characteristics to aid the selection of patients for whom chemotherapy may be appropriate based on prognosis. Several of these signatures are commercially available, including the Oncotype Dx recurrence score (RS) (Genomic Health), PAM50-based Prosigna risk of recurrence (ROR) (NanoString), Breast Cancer Index (BCI) (bioTheranostics), EndoPredict (EPclin) (Myriad Genetics), and MammaPrint Netherland Kanker Institute 70-gene signature (Agendia BV), are endorsed by several guidelines and are routinely used by clinicians.
In the present study, a within-patient comparison of the prognostic value of 6 multigene signatures in women with early ER-positive breast cancer who received endocrine therapy for 5 years was conducted. The retrospective biomarker analysis included 774 postmenopausal women with ER-positive ERBB2 (formerly HER2)–negative breast cancer. This analysis was performed as a preplanned secondary study of data from the  Anastrozole or Tamoxifen Alone or Combined randomized clinical trial comparing 5-year treatment with anastrozole vs tamoxifen with 10-year follow-up data (see the study protocol and trial registration ISRCTN18233230). The analysed signatures included the Oncotype Dx recurrence score, PAM50-based Prosigna risk of recurrence (ROR), Breast Cancer Index (BCI), EndoPredict (EPclin), Clinical Treatment Score, and 4-Marker Immunohistochemical Score. Data were collected from January 2009, through April 2015.
Anastrozole or Tamoxifen Alone or Combined randomized clinical trial comparing 5-year treatment with anastrozole vs tamoxifen with 10-year follow-up data (see the study protocol and trial registration ISRCTN18233230). The analysed signatures included the Oncotype Dx recurrence score, PAM50-based Prosigna risk of recurrence (ROR), Breast Cancer Index (BCI), EndoPredict (EPclin), Clinical Treatment Score, and 4-Marker Immunohistochemical Score. Data were collected from January 2009, through April 2015.
Related articles across the web
 Men can get breast cancer too. Let’s add blue to the pink ribbon
Men can get breast cancer too. Let’s add blue to the pink ribbon Prostate cancer overtakes breast cancer to become third biggest killer – signs of disease
Prostate cancer overtakes breast cancer to become third biggest killer – signs of disease National Cancer Institute Awards $3.3 Million 5-Year Academic-Industry Grant to Inspirata and Case Western Reserve University for Breast Cancer Risk Assessment Assay
National Cancer Institute Awards $3.3 Million 5-Year Academic-Industry Grant to Inspirata and Case Western Reserve University for Breast Cancer Risk Assessment Assay Winter 2018 News Briefs
Winter 2018 News Briefs Life after cancer is anything but ordinary
Life after cancer is anything but ordinary
Related posts
In Caribbeans cystic fibrosis (CF) is driven by very rare CFTR mutations
Huge step forward for HealthKit, iOS, iPhone, AppleWatch: FDA permits marketing ...
FDA safety communication concerning E-cigarettes
Genetic predisposition and sweating: Most Koreans don’t have to wear deodorants
Sodium-glucose cotransporter-2 (SGLT2) inhibitors in Type-2 Diabetes: Risk of ac...
Ph.D.; Professor in Pharmacology and Toxicology. Senior expert in theragenomic and personalized medicine and individualized drug safety. Senior expert in pharmaco- and toxicogenetics. Senior expert in human safety of drugs, chemicals, environmental pollutants, and dietary ingredients.


If you are going for finest contents like I do, just
pay a visit this web page everyday for the reason that it
gives quality contents, thanks