A very exciting week for patients with hepatitis C viral (HCV) infections of the genotypes 3 and 4
Last Updated on July 31, 2015 by Joseph Gut – thasso
July 31, 2015 – This week, just in time for World Hepatitis Day on July 28, the American Food and Drug Administration (FDA) approved Daclatasvir (Daklinza) for use with sofosbuvir to treat hepatitis C virus (HCV) genotype 3 infections. In addition, the FDA approved a fixed combination of Ombitasvir with Paritaprevir and Ritonavir (Technivie) for use in combination with ribavirin for the treatment of hepatitis C virus (HCV) genotype 4 infections, particularly for patients without scarring and poor liver function (i.e., cirrhosis).
Hepatitis C is a viral disease that causes inflammation of the liver that can lead to diminished liver function or liver failure. Most people infected with HCV have no symptoms of the disease until liver damage becomes apparent, which may take several years. Some people with chronic HCV infection develop scarring and poor liver function (cirrhosis) over many years, which can lead to complications such as bleeding, jaundice (yellowish eyes or skin), fluid accumulation in the abdomen, infections or liver cancer. According to the Centers for Disease Control and Prevention, approximately 2.7 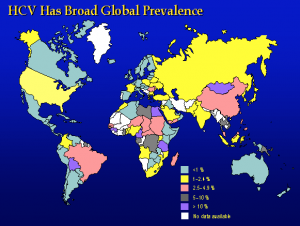 million Americans are infected with HCV of which approximately 10 percent are genotype 3, while genotype 4 seems one of the least common types of the HCV infections. Worldwide however, according to the World Health Organisation (WHO), there is a total of about 130 to 150 million patients suffering from HCV, irrespective of viral genotype, and about 500’000 patients are dying from it every year.
million Americans are infected with HCV of which approximately 10 percent are genotype 3, while genotype 4 seems one of the least common types of the HCV infections. Worldwide however, according to the World Health Organisation (WHO), there is a total of about 130 to 150 million patients suffering from HCV, irrespective of viral genotype, and about 500’000 patients are dying from it every year.
According to FDA, Daklinza is the first drug that has demonstrated safety and efficacy to treat genotype 3 HCV infections without the need for co-administration of interferon or ribavirin, two FDA-approved drugs also used to treat HCV infection. The safety and efficacy of Daklinza in combination with sofosbuvir were evaluated in a clinical trial of 152 treatment-naive and treatment-experienced participants with chronic HCV genotype 3 infection. Participants received Daklinza 60 mg plus sofosbuvir 400 mg once daily for 12 weeks and were monitored for 24 weeks post treatment. Results showed that 98 percent of the treatment-naive participants with no cirrhosis of the liver and 58 percent of the treatment-naive participants with cirrhosis achieved sustained virologic response. Of the participants who were treatment-experienced, 92 percent with no cirrhosis of the liver and 69 percent with cirrhosis achieved sustained virologic response. Daklinza labeling carries a Limitations of Use statement to inform prescribers that sustained virologic response rates are reduced in HCV genotype 3 infected patients with cirrhosis.
Likewise, Technivie in combination with ribavirin is the first drug that has demonstrated safety and efficacy to treat genotype 4 HCV infections without the need for co-administration of interferon, an FDA-approved drug also used to treat HCV infection. The safety and efficacy of Technivie with ribavirin were evaluated in a clinical trial of 135 participants with chronic HCV genotype 4 infections without cirrhosis. Ninety-one participants received Technivie with ribavirin once daily for 12 weeks. Forty-four participants received Technivie once daily without ribavirin for 12 weeks. The studies were designed to measure whether a participant’s hepatitis C virus was no longer detected in the blood 12 weeks after finishing treatment (sustained virologic response), suggesting a participant’s infection had been cured. Results showed that 100 percent of the participants who received Technivie with ribavirin achieved a sustained virologic response. Of those who received Technivie without ribavirin, 91 percent achieved sustained virologic response.
These treatment options, now also for patients with HCV infections of genotypes 3 and 4 are very assuring. The real world questions however, remains unanswered: Who will have access and can afford to these most useful drugs? The problem here is pricing. Who is going to pay the treatment for all the patients worldwide, where neither the patients nor the (perhaps inexistent) health care system has the resources to serve the extraorbitant price tags cited by some of the makers of these novel anti-HCV drugs (see also this article from thasso post)? Remember that the majority for HCV-patients does not reside in the US and economical, clinical, and regulatory conditions very different from those in the US may apply for these patients.


Looking great work dear, I really appreciated to you on this quality work. Nice post!! these tips may help me for future. Thanks for the Illinois Custody Agreements and Parenting Plans | World entry, thasso.com webmaster! For more information on mesothelioma treatment options refer to Lukas(%URL%) website and derive more information benefit.