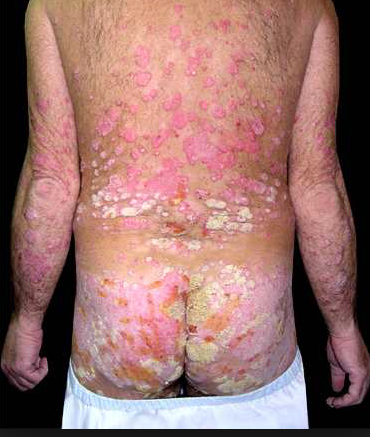
Ixekizumab (Taltz) for plaque psoriasis: Positive opinion by the European CHMP
Last Updated on March 4, 2016 by Joseph Gut – thasso
March 04, 2016 – On February 25, 2016, the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending the granting of a marketing authorisation for the medicinal product Ixekizumab (Taltz), intended for the treatment of plaque  psoriasis. Ixekizumab (Taltz) will be available as a 80 mg solution for injection. The active substance of Taltz, Ixekizumab, is an immunosuppressant. Ixekizumab is a monoclonal antibody that binds with high affinity and specificity to both forms of interleukin 17A (IL‑17A and IL‑17A/F). Neutralisation of IL‑17A by Ixekizumab inhibits keratinocyte proliferation and activation which have been implicated in the pathogenesis of psoriasis.
psoriasis. Ixekizumab (Taltz) will be available as a 80 mg solution for injection. The active substance of Taltz, Ixekizumab, is an immunosuppressant. Ixekizumab is a monoclonal antibody that binds with high affinity and specificity to both forms of interleukin 17A (IL‑17A and IL‑17A/F). Neutralisation of IL‑17A by Ixekizumab inhibits keratinocyte proliferation and activation which have been implicated in the pathogenesis of psoriasis.
The benefits with Ixekizumab (Taltz) are its statistically significant and clinically relevant effects compared to placebo or etanercept in terms of ‘Psoriasis Area and Severity Index’ (PASI) score 75 and ‘static Physician Global Assessment of 0 or 1 (sPGA (0/1)) at week 12. PASI 90, PASI 100 and sPGA 0 response rates indicating nearly complete/complete clearance were also statistically significantly better with Taltz compared to placebo or Etanercept (Enbrel).
The most frequently reported adverse drug reactions were upper respiratory tract infections. Most of the reactions were mild or moderate in severity.The full indication is: “Taltz is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy”. It is proposed that Taltz is prescribed by physicians experienced in the treatment and diagnosis of psoriasis.
Detailed recommendations for the use of this product will be described in the summary of product characteristics (SmPC), which will be published in the European public assessment report (EPAR) and made available in all official European Union languages after the marketing authorisation has been granted by the European Commission.
The mode of action of Ixekizumab (Waltz) is very similar if not identical to that of Secukinumab (Cosentyx), which has earlier been approved by both the American Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of plaque psoriasis as well. Both, Ixekizumab and Secukinumab are monoclonal antibodies that bind and inhibit IL 17A.


This is very interesting, You’re a very skilled blogger.
I’ve joined your rss feed and look forward to seeking more
of your wonderful post. Also, I have shared your web site
in my social networks!
Hey, It’s a helpful site.
This article Ixekizumab (Taltz) for plaque psoriasis: Positive opinion by the European CHMP, So helpful for taltz psoriasis! I wanna share this post to my site, can I?
The CHMP recommends the granting of a marketing authorisation for the IL-17A inhibitor Ixekizumab (Waltz) for the treatment of plaque psoriasis
Please, do so. You are welcome.
The FDA has just approved Ixekizumab (Taltz) to treat adults with moderate-to-severe plaque psoriasis. Read on here:
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm