Cabometyx and Kisplyx: Two new medicines for advanced kidney cancer
Last Updated on July 27, 2016 by Joseph Gut – thasso
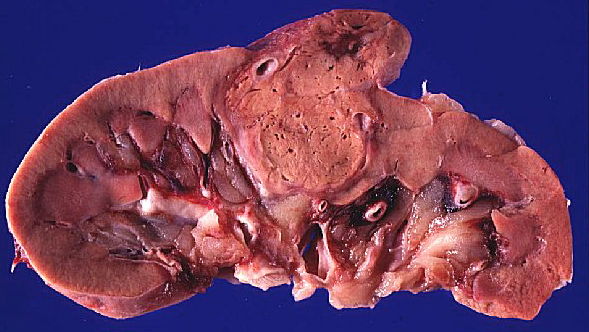
July 26, 2016 – Renal cell carcinoma is the most common form of kidney cancer in adults. Advanced renal cell carcinoma includes both metastatic disease and locally advanced renal cell carcinoma that cannot be removed by surgery. Despite the recent approval of new therapies for advanced renal cell carcinoma, many patients who do not respond to the existing treatments have a poor prognosis. Therefore new treatment options are needed.
 In the light of this situation, on the last meeting of the Committee for Medicinal Products for Human Use (CHPM) in July 2016, the European Medicines Agency (EMA) has just recommended granting marketing authorisations in the European Union (EU) for two medicines, namely Cabozantinib (Cabometyx) and Lenvatinib (Kisplyx), for the treatment of advanced renal cell carcinoma (kidney cancer). Both are indicated for the treatment of adult patients with advanced renal cell carcinoma who have been previously treated with a vascular endothelial growth factor (VEGF)-inhibitor; Cabozantinib (Cabometyx) is to be used as monotherapy while Lenvatinib (Kisplyx) is for use in combination with Everolimus (Afinitor, see also the EPAR on Afinitor here).
In the light of this situation, on the last meeting of the Committee for Medicinal Products for Human Use (CHPM) in July 2016, the European Medicines Agency (EMA) has just recommended granting marketing authorisations in the European Union (EU) for two medicines, namely Cabozantinib (Cabometyx) and Lenvatinib (Kisplyx), for the treatment of advanced renal cell carcinoma (kidney cancer). Both are indicated for the treatment of adult patients with advanced renal cell carcinoma who have been previously treated with a vascular endothelial growth factor (VEGF)-inhibitor; Cabozantinib (Cabometyx) is to be used as monotherapy while Lenvatinib (Kisplyx) is for use in combination with Everolimus (Afinitor, see also the EPAR on Afinitor here).
Both Cabozantinib (Cabometyx) and Lenvatinib (Kisplyx) are tyrosine kinase inhibitors ((TKIs); for a overview of TKIs see here). This means that they work by blocking certain enzymes known as tyrosine kinases. These enzymes can be found in some receptors on the surface of cancer cells and are involved in the growth and spread of cancer cells, and in the blood vessels that supply the tumours.
Both active substances are already approved in the EU as different medicines for the treatment of thyroid cancer, an orphan condition. In December 2013, EMA recommended for approval another cabozantinib-containing medicine (Cometriq) to treat adults with medullary thyroid cancer. Another lenvatinib-containing medicine (Lenvima) was recommended for approval for the treatment of patients with thyroid carcinoma in March 2015.
The main study on which Cabozantinib (Cabometyx)’s recommendation is based is a phase III trial involving 658 patients with metastatic renal cell carcinoma that had progressed after prior VEGF receptor tyrosine kinase inhibitor therapy. In this study, patients treated with Cabozantinib (Cabometyx) had a longer period of time without their disease progressing (progression-free survival) compared to patients treated with Everolimus (Afinitor). (7.4 months compared to 3.8 months). In addition, preliminary results showed that patients treated with Cabozantinib (Cabometyx) lived longer than patients treated with Everolimus (Afinitor). alone (median of 21.4 months compared to 16.5 months). The most frequent adverse reactions associated with Cabozantinib (Cabometyx) include diarrhoea, fatigue, nausea, decreased appetite, palmar-plantar erythrodysaesthesia syndrome (hands and feet redness, swelling and pain), hypertension and vomiting. The Committee for Medicinal Products for Human Use (CHMP) concluded that the benefit/risk balance of Cabozantinib (Cabometyx) is positive.
The main study on which Lenvatinib (Kisplyx)’s recommendation is based is a Phase Ib/II trial involving 153 patients with metastatic or unresectable renal cell carcinoma who received at least one prior VEGF targeted therapy and were treated either with the combination of Lenvatinib (Kisplyx) with Everolimus (Afinitor). or with one of these agents. In this study, patients’ progression-free survival was 12.8 months on average for patients treated with Lenvatinib (Kisplyx) in combination with Everolimus (Afinitor)., compared to 5.6 months for patients treated with Everolimus (Afinitor). alone based on independent review of radiological images. In addition, encouraging signs of prolonged overall survival were seen in patients treated with the combination therapy. The most frequent adverse reactions include diarrhoea, fatigue, decreased appetite, vomiting, nausea and hypertension. Severe diarrhoea occurred at a higher frequency in the combination group than in the Everolimus (Afinitor). The CHMP considered that the benefits of Lenvatinib (Kisplyx) in combination with Everolimus (Afinitor). outweigh its risks but requested that post-authorisation studies be conducted to collect further data on the safety and efficacy of the combination therapy to complement data from the phase Ib/II trial.

